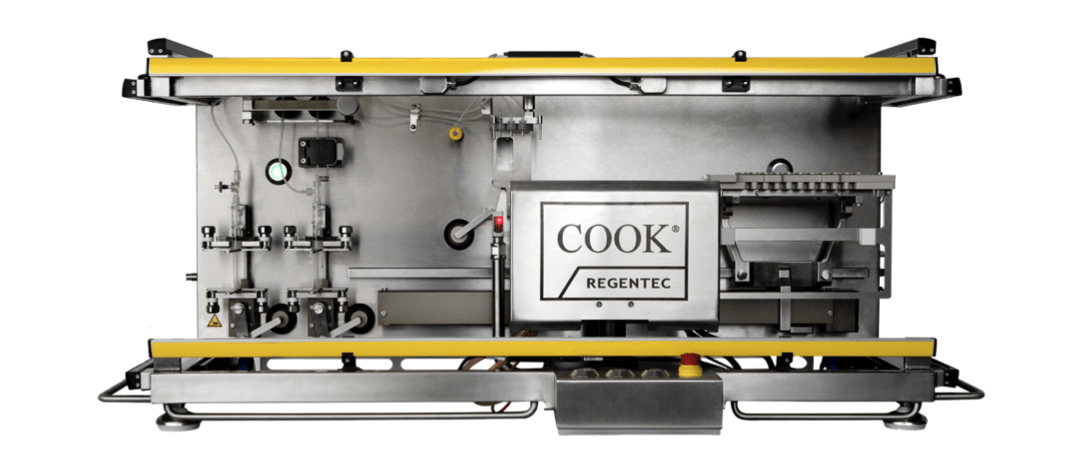
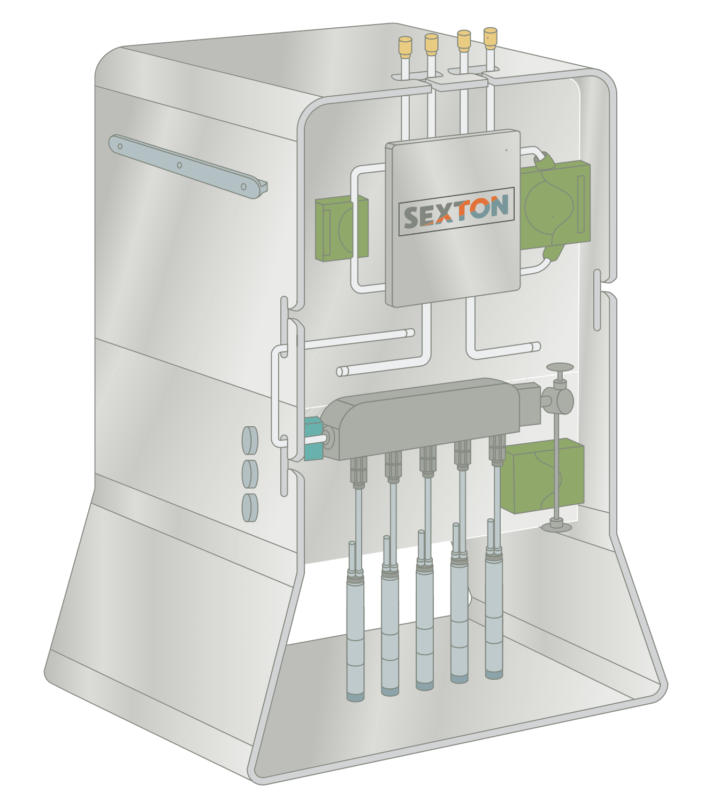
Sexton Biotechnologies, part of BioLife Solutions Inc., provides products and services to those creating cell and gene therapies that will save and improve the lives of our patients. Stemming from the Medieval Latin, the Sexton was to be wise, humble, and committed to serving the needs of their community. Our products perform to both protect and enhance the innovative work being done in labs across the world to accelerate the path toward clinical success and provide a better life for our patients.