The XELTA 2D bags are designed to meet the demands of crucial applications in the biopharmaceutical and pharmaceutical industries. These bags are reliable, flexible, and easy to install, offering advantages in terms of product recovery and ease of use. Manufactured from premium film with a thickness of 0.325mm, the XELTA 2D bags are validated for use in manufacturing processes. They are available in a wide range of sizes and are suitable for various applications such as storage, transport, and filtering of biopharmaceutical fluids. The bags can be customized with connectors, tubing, and clamps to meet specific process requirements. Manufactured in accordance with ISO 9001:2015 certified quality management systems and cGMP requirements, the XELTA 2D bags undergo extensive testing to ensure compliance with pharmacopoeial standards and bio-compatibility. The specialized film used in these bags provides good resistance to chemicals and polar solvents, with favorable properties such as clarity, tensile strength, and gas permeability.

The XELTA 2D bags have been designed for crucial applications where systems are required to be reliable, flexible, and easy to install. Manufactured from premium film and with a film thickness of 0.325mm the XELTA 2D bags are validated for use in the biopharmaceutical and pharmaceutical manufacturing processes. The XELTA 2D bags bring together state-of-the-art design features that improve the robustness of single-use systems. This brings considerable advantages in terms of ease of use and product recovery when dealing with biopharmaceutical fluids.
The XELTA 2D bags are designed to meet the demands of crucial applications in the biopharmaceutical and pharmaceutical industries. These bags are reliable, flexible, and easy to install, offering advantages in terms of product recovery and ease of use. Manufactured from premium film with a thickness of 0.325mm, the XELTA 2D bags are validated for use in manufacturing processes. They are available in a wide range of sizes and are suitable for various applications such as storage, transport, and filtering of biopharmaceutical fluids. The bags can be customized with connectors, tubing, and clamps to meet specific process requirements. Manufactured in accordance with ISO 9001:2015 certified quality management systems and cGMP requirements, the XELTA 2D bags undergo extensive testing to ensure compliance with pharmacopoeial standards and bio-compatibility. The specialized film used in these bags provides good resistance to chemicals and polar solvents, with favorable properties such as clarity, tensile strength, and gas permeability.
The XELTA 2D bags offer users the flexibility to order a wide range of configurations. You can choose from a diverse selection of connectors, tubing, clamps, and other components from any supplier to customize the bags according to your specific process requirements.
Our XELTA 2D bags are meticulously designed, developed, and manufactured in strict adherence to an ISO 9001:2015 certified Quality Management System. Prior to shipping, these bags undergo rigorous testing to ensure their quality and reliability. We take great care to manufacture our products in compliance with cGMP requirements, making them suitable for cleanroom processes.
MORE SOLUTIONS
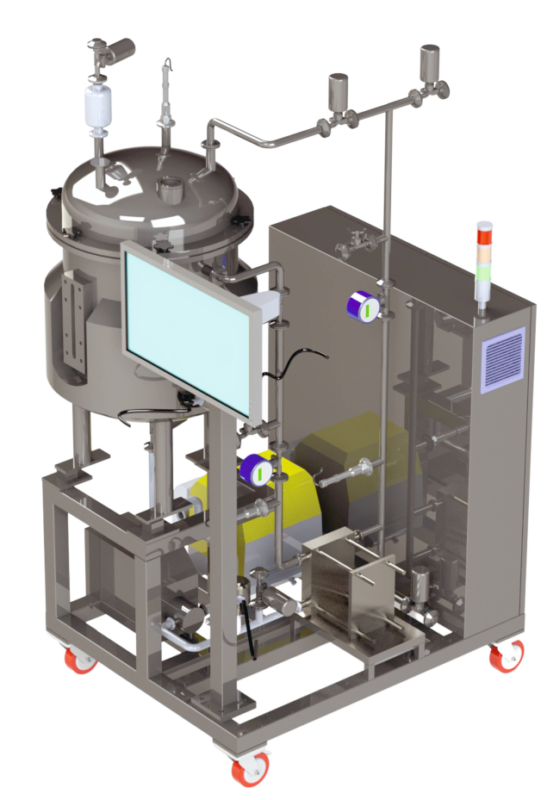
NXTtff
The NXT TFF system by PharmNXT Biotech is a system that’s perfect for running fully automated UF/DF processes at high capacity. It can handle any volume between 20L and 500L (200 g to 2.5 kg), and the compact system, has a low hold-up volume and the capacity to accommodate cassettes with surface area ranging from 0.5 to 2.5 m2

Chromatography Resins (Purolite)
The purification of commercially-available monoclonal antibodies (mAbs) on the market today is typically performed in three chromatography steps. Purolite offer industry-leading solutions for each step.
Protein A Affinity Chromatography
A Protein A affinity resin is utilized as an initial antibody capture step. Praesto Protein A resins deliver exceptionally high purity (>99%) and yield in a single, efficient step.
Cation Exchange Chromatography
A Sulphopropyl- (SP) functionalized cation exchange resin in ‘bind and elute’ mode is used to remove aggregates and HCP (Host Cell Proteins).
Anion Exchange Chromatography
A Quarternary Amine (Q) functionalized anion exchange resin is used in ‘flow-through’ mode to remove trace contaminates and ensure sufficient viral clearance.

NXTmfilt
Single-use technology continues to be adopted for many areas of bioprocessing. The use of automation provides additional benefits in manufacturing such as consistency in product quality, reduced labor costs, and reduction of operator errors.

